If your pharmacist says OTC medications are sufficient to treat your condition you should ask which products provide FDA-approved effective treatment. New Drug Application NDA For an NDA the company writes and submits an application which includes thousands of pages to the FDA for review and approval.
 From Molecule To Medicine Cabinet A Drug S Long Journey From Development To Approval
From Molecule To Medicine Cabinet A Drug S Long Journey From Development To Approval
FDA registration for cosmetic products is not mandatory FDA does not certify or approve cosmetic products.

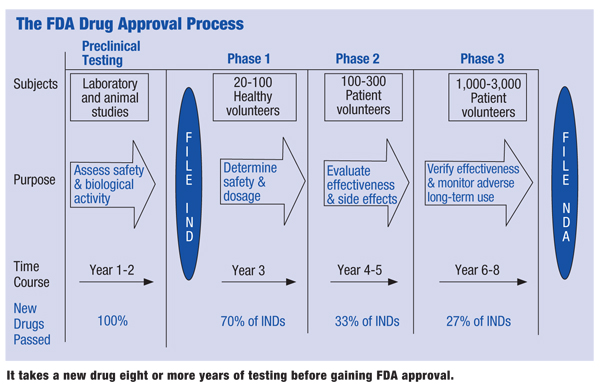
How to obtain fda approval. Phase III The goal of a Phase III study is to test how the new treatment compares with the standard treatment. FDA batch certification is required for certain color additives. The NDA is the official request for US approval of a drug.
In the midst of the pandemic the vaccine manufacturers chose to first apply for FDA emergency use authorization before applying for FDA approval. LMG will issue a certificate of FDA registration to our clients for their records at free of cost. For more information about how to get FDA certification or FDA approval please contact us at infofdahelpus.
During this step of the drug approval process the drug sponsor will formally ask the FDA to approve a drug for marketing in the US. FDA review the sample of each lot of color additive manufactured and certify if it complies with requirement. Coronavirus Updates The agencys approval of the Pfizer-BioNTech vaccine shown to be 95 effective would go beyond the speedier and.
The FDA registration number only recognizes that your establishment is registered with US FDA. The names and qualifications of the RDRC members and consultants 3611 c 4 a statement that the RDRC agrees to comply with the. Complete FDA 2914 including.
To get FDA approval drug manufacturers must conduct lab animal and human clinical testing and submit their data to FDA. Human drugs and therapeutic biologicals proteins and other products derived from living sources used for therapeutic purposes Drug Approval Reports by Month. Once all of that is complete -- both the clinical trials and manufacturing details -- companies can submit a Biologics License Application or BLA to the FDA.
Then the review process starts. Unlike premarket notification PMA approval is to be based on a determination by FDA that the PMA contains sufficient valid. Drug approval packages are available on the FDA website for drugs approved since 1997.
Pfizer Seeks Full FDA Approval For COVID-19 Vaccine. 7 22 Data on drugs approved before 1997 can be requested at the following link. After completion of a Phase II trial a new treatment usually must go through a Phase III trial in order to obtain FDA approval.
Manufacturers decide whether and when to submit a request for authorization or for approval an FDA spokesperson said in a statement to HuffPost. The application will also include how the drug behaves in the body and the manufacturing process. By submitting a BLA to the FDA.
The NDA includes all animal and human data plus side effects dosing and effectiveness. The US FDA will assign a unique registration number to each registered food facility the assignment of FDA registration number does not denote the approval of your establishment or product by the US FDA. An EUA request for a COVID-19 vaccine can be submitted to FDA based on a final analysis of a phase 3 clinical efficacy trial or an interim analysis of.
FDA will then review the data and may approve the drug if the agency determines that the benefits of the drug outweigh the risks for the intended use. A PMA is an application submitted to FDA to request approval to market. Our clients and their.
By submitting an NDA. You can also ask whether the product is. The new drug application includes all animal and human data and an analysis of the data.
Most Importers and Shipping companies always ask for FDA registration certificate or proof of FDA registration to the manufacturer.